Join Current Studies
Join a study to learn more about your condition, explore new treatments, and contribute to medical advancements.

DBC-PUL-02
The purpose of this clinical investigation is to assess safety and performance of Compedica’s OptiPulse™ (also known as PulseFlowDF™) and to collect subject outcome data on the treatment of diabetic foot ulcers (DFUs) versus Standard of Care (SOC). OptiPulse™ is designed to enhance blood circulation in the venules and arterioles

ETS-MG-DFU-02
The purpose of this clinical evaluation is to collect patient outcome data on a commercially available 510K FDA cleared synthetic, absorbable skin substitute matrix. The commercially available product is MIRRAGEN™ Advanced Wound Matrix and consists of a borate-based bioactive glass fiber and particulate,
versus Standard of Care (SOC)
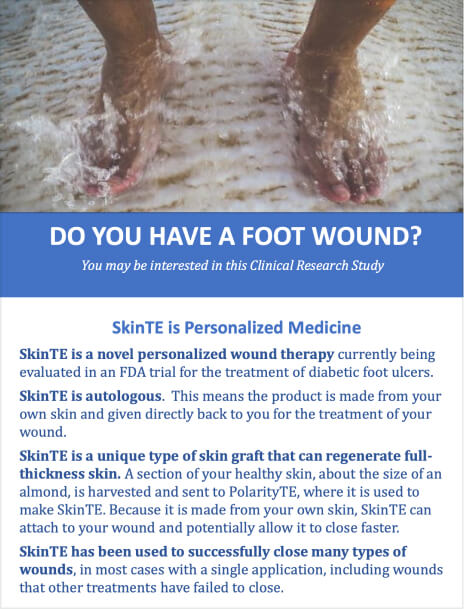
COVER's DFU:
This clinical trial is being conducted to determine if SkinTE can substantially increase the wound healing trajectory of DFUs. Therefore, collection of human outcomes data is necessary to quantify this effect under controlled conditions in which study subjects are treated with one application of SkinTE plus SOC, or SOC alone.

EX6018-4758
The primary objective is to demonstrate the superiority of ziltivekimab 15 mg s.c. once-monthly in reducing the risk of MACE (as defined by the primary endpoint) compared to placebo, both added to standard of care, in participants with established ASCVD, CKD and systemic inflammation

VP-VLY-686-33-01
To evaluate the efficacy of tradipitant relative to placebo in change from baseline to Week 12 in daily nausea severity scores.

CIN-102-123
The primary objective of this study is to assess the ability of CIN-102 to significantly decrease the severity of gastroparesis-related symptoms as compared to baseline in adult subjects with diabetic gastroparesis, , compared to placebo.